
Graves’ Disease
Graves’ disease is an immune system disorder in which abnormal antibodies are produced that are directed toward parts of the body, primarily the thyroid gland and the eye areas. They typically cause over action of the thyroid gland, resulting in elevated heart rate, anxiety, tremors, and weight loss, among other symptoms. When the antibodies affect the eye areas, there can be swelling and retraction of the eyelids, redness, protrusion and crossing of the eyes, double vision, and even severe vision loss and blindness.
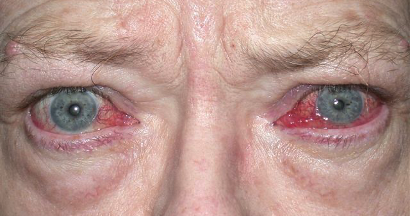

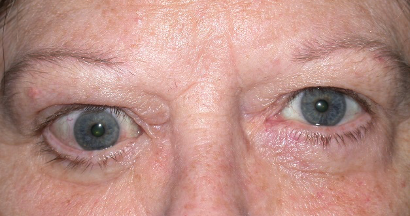

There is no medical therapy that can eliminate the source of the problem, the abnormal antibodies. The overactive thyroid gland is treated by suppressing or destroying it, and then taking a replacement thyroid hormone pill. We obviously can’t just destroy and replace eyes that are affected by the antibodies in Graves’ disease. Traditionally, we have used eye drops, steroid pills, and radiation therapy to try and suppress the inflammation of the eyes caused by the autoantibodies. Then once the condition naturally subsides and becomes inactive (which may be after 6 months to two years), we would do surgeries to correct the eyelids, eyeball protrusion, or double vision.
A new option is now available.
Teprotumumab (Tepezza, Horizon Therapeutics) was approved by the FDA in January 2020 for the treatment of Graves’ Eye Disease. It is a human monoclonal antibody that blocks the receptors targeted by the autoantibodies in Graves’ Eye Disease, thereby limiting the inflammation around the eyes. The drug is given as an IV infusion once every 3 weeks for 24 weeks. It has been shown to reduce the amount of eyeball protrusion (proptosis), and also appears to help with the swelling, redness, and double vision. Teprotumumab is generally indicated for more severe and active cases of Graves’ Eye Disease, and we are seeing positive results.
Recent Comments